Chapter 2 – Carbon Dioxide Is Not a Pollutant
By Mike Belsick
While it is true that increasing industrialization is generating more carbon dioxide over pre-industrial ambient levels, the increase is a very modest percentage. Keep in mind that the industrialized burning of fossil fuels adds two different components to the atmosphere: soot or other particles and carbon dioxide. It is the soot that causes smog. Carbon dioxide is invisible. However, the Left talks as though carbon dioxide (CO2) is a pollutant formed from burning fossil fuels. That is a very misleading description. Another source of CO2 is you. In every exhaled breath of air, you put slightly more CO2 in the atmosphere than what you breathed in. That is called respiration and every living animal on Earth does exactly the same thing. We need oxygen (O2) to survive – to power the engines inside our bodies. The byproduct of our internal engines is CO2. Essentially, every human is like an automobile. We need fuel and air to run. We also create byproducts that we do not need. Is our exhaled breath air pollution? Consider this, while animals give off CO2, plants need that CO2 to grow. In the process of photosynthesis, plants use CO2, water, a few minerals and organic matter, in the presence of sunlight to grow. The byproduct of a plant’s internal engine is oxygen. This is a perfect symbiotic relationship between plants and animals. Since all animal life on Earth is dependent directly or indirectly on plants and plants can only grow with CO2, carbon dioxide should never be considered a pollutant. Just like oxygen, CO2 is critical for all life on Earth. In fact, satellite imagery shows a much greener planet thanks to the current slightly higher levels of carbon dioxide in the atmosphere. Look at it this way. While Alexandria Ocasio-Cortez’s “Green New Deal” wants to illuminate CO2, slightly increasing levels of CO2 is making the Earth far greener. Isn’t that ironic? Maybe AOC should call her plan the “Brown New Planet”.
What is the composition of air? By volume, dry air contains 78.09% of nitrogen, 20.95% of oxygen, 0.93% of argon, 0.03% of carbon dioxide, and small traces of other gases, as shown in Figure 4.
Please note the incredibly small percentage of CO2 that is normally in the atmosphere. We know from deep ice core samples in Antarctica that the concentration of CO2 in the atmosphere constantly changed up and down over the last 800,000 years. On the average, CO2 is about 250 ppm (parts per million) with peaks around 300 ppm. Currently, it is said that CO2 levels are about 400 ppm. So instead of normal CO2 levels being around 0.03% of the atmosphere, it is currently around 0.04%. That represents a change of 0.01% of the atmosphere. Could a change that small wipe out all life on Earth? Very doubtful.
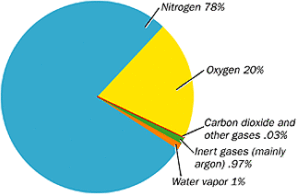 Figure 4. Composition of Air
Figure 4. Composition of Air
Water vapor is also a component of normal air, but it varies a lot due to conditions such as temperature and location. Water vapor can be as high as 4% (100 times that of CO2) in tropical regions. On the average, water vapor would be between 2% to 3% of the atmosphere. Carbon dioxide and water vapor are considered as the main components of “greenhouse gases” that blanket the Earth to keep it warm at night. Again, please note that water vapor, as a percentage of what is considered as a greenhouse gas, is significantly higher than carbon dioxide. As such, water vapor has a much larger impact on the climate than carbon dioxide ever can. However, no “man-made climate change believer” discusses water vapor. All the greenhouse gases are critical for life on Earth to keep us warm. On average, these two gases together only amount to 2-3% of the total atmosphere. It is amazing, in a very positive way, that such a very small percentage of air can have such a beneficial bearing on life on Earth by keeping us from freezing at night.
When sunlight first hits the Earth’s outer atmosphere, some of the sun’s energy is bounced back into space, about 30%. Some of this energy is absorbed by the atmosphere, about 20%. The other half of the sun’s energy is absorbed by the Earth and warms it. At night, when there is no more heat from the sun, the heat absorbed by the Earth wants to radiate back into cold space. However, the atmosphere, specifically greenhouse gases, traps some of this heat from escaping. Water vapor and carbon dioxide prevent us from freezing at night. In a later chapter, I will go more into this energy balance.
Just to end this chapter with an important fact. The average Earth temperature (everywhere on Earth at the same time), if it did not have an atmosphere with greenhouse gases, would be -0.40 F (-180 C). That would be well below freezing just like the planet Mars that has very little atmosphere. Instead, the average temperature is 590 F (150 C) which is very habitable. Greenhouse gases (water vapor and carbon dioxide) make this possible. Instead of killing the planet Earth, carbon dioxide and water vapor are making it livable.
To be fair to the man-made climate change believers and to be honest about the presentation of data, there is one chart, Figure 5, that man-made climate change believers like to rely upon. It supposedly shows the correlation between CO2 concentrations and Earth temperature.
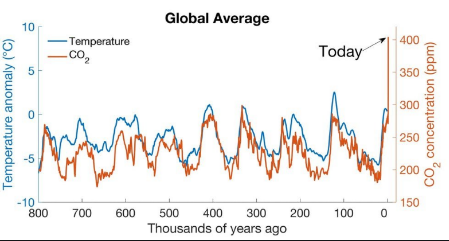 Figure 5. Correlation of Earth Temperature and CO2
Figure 5. Correlation of Earth Temperature and CO2
At first glance, there appears to be some relationship between the two measures. It would be easy to imagine how someone could become worried about a drastic rise of CO2 ending up causing a significant rise of the Earth’s temperature. However, there are some questions to consider before someone starts running around saying the “sky is falling” (reference to Chicken Little).
-
- Deep Antarctic core samples supposedly can determine CO2 concentrations back 800,000 years. While I can imagine how ancient samples of air, including CO2, could be trapped in years upon years of falling snow, how do you know what the temperature was over this 800,000-year period? (See next chapter on Milankovitch Cycles)
- As one pulls up these core samples from Antarctic, how do you know how old the sample of CO2 is? Carbon dating is the normal process of determining the age of something, but that is only valid for 75,000 years ago and not 800,000 years ago. So how do you know that the last sample pulled up is 800,000 years old? I am speculating that the age is merely an educated guess. See chapter 4.
- However one determines or guesses what the CO2 concentration was, when it was that concentration, and what the temperature was at that time, the next question to ask is which one (temperature or CO2 concentration) is the cause of the other (CO2 concentration or temperature) or is this merely a coincidence with something else causing both to react in a similar manner?
As persuasive as this chart may be used to prove an idea, there are too many questions in my mind that are unanswered to consider this chart as persuasive. Naturally, no one of the man-made climate change believers is addressing these questions. Why should they when 97% of all scientists believe in anthropogenic climate change.
In summary, CO2 is not a pollutant but rather is critical for humans and animals to exist. CO2 concentrations and temperatures have fluctuated throughout hundreds of thousands of years yet it is not certain if the changes are the results of one acting on the other or which order. CO2 concentration has increased since industrialization and currently is about 0.04% of our atmosphere (from pre-industrialization high of 0.03%). Keep in mind that water vapor, the other greenhouse gas, ranges from near zero in incredibly dry places to around 4% of the atmosphere in incredibly wet places.
